Only electrons are involved in chemical reactions (breaking and making bonds) – we don’t turn atoms into different atoms, so nuclei remain unchanged. It is therefore the arrangement of the electrons in their shells that gives an atom or ion its chemical properties.
Bohr’s model of electronic structure
Bohr’s model stated that electrons only have fixed amounts of energy, which means they are confined to specific energy levels, often called shells. Each shell has a maximum number of electrons it can contain.
Electrons can move from one energy level to another, but not exist anywhere other than in an energy level. When they move, they absorb or emit the difference in energy between the energy levels. The further from the nucleus a shell is, the higher its energy is.
The contributions of Quantum Mechanics:
The currently accepted model of the atom makes use of quantum mechanics to build on Bohr’s model, adding further detail and sophistication to allow additional phenomena to be explained:
- Electrons occupy defined regions of space called orbitals. An orbital can only contain 0, 1 or 2 electrons, never more. Along with being negatively charged, electrons have a momentum-related property called spin. We denote the spin of the first electron in an orbital as ‘up’ (shown as ↑) and a second electron in an orbital then has ‘down’ spin (↓). To share an orbital, electrons must have opposite spin.
Definition: An orbital is a region of space that can hold up to two electrons, each having opposite spins.
- There are different types of orbitals, with specific shapes.
- Orbitals always come in sets – called subshells. A subshell is a set of orbitals of the same type.
The illustration below shows the shapes of the orbitals that make up the s, p, d and f subshells. N.B. For A level we only need to learn the shapes of s and p orbitals.
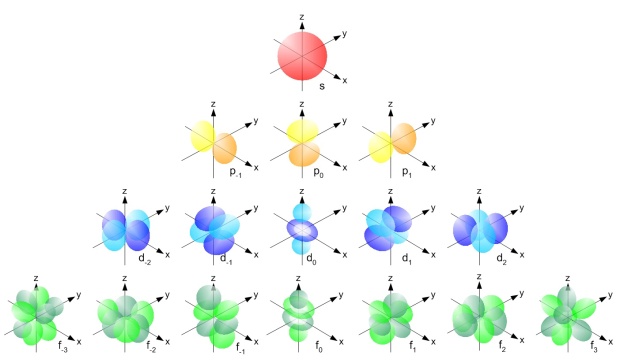
Source: used under Creative Commons licence
an s-subshell = one s-orbital (so it can contain up to 2 electrons)
a p-subshell = three p-orbitals (so can contain up to 6 electrons)
a d-subshell = five d-orbitals (so can contain up to 10 electrons)
an f-subshell = seven f-orbitals (so can contain up to 14 electrons)
- Each shell is labelled with a number – the principle quantum number, n. The innermost shell is n=1, and so on outwards. Shells consist of a set of subshells.
| Shell | Comprised of subshells | Maximum electrons |
| n=1 | 1s | 2 |
| n=2 | 2s, 2p | 2 + 6 = 8 |
| n=3 | 3s, 3p, 3d | 2 + 6 + 10 = 18 |
| n=4 | 4s, 4p, 4d, 4f | 2 + 6 + 10 + 14 = 32 |
Filling Rules:
We know how many electrons an atom has, from the atomic number. We now need rules to tell us how these electrons are arranged in the shells, subshells and orbitals in the ground state (the lowest atomic energy, when none of the electrons have been excited to higher energy levels, which is the normal state for the atom)
RULE 1) An electron will go into the lowest energy subshell that is not already filled.
What determines the energy of a subshell is how far away from the nucleus (on average) the electrons are in that subshell – the nearer to the nucleus, the lower the energy of the subshell. Within a shell, the s-subshell is lower in energy than the p-subshell, and p-subshell is lower in energy than the d-subshell etc.
Across the shells there are some surprises because of the different shapes of the orbitals e.g. the electrons in the 4s subshell are on average nearer the nucleus than those in 3d, and so lower in energy than 3d. Which means the 4s subshell will fill before the 3d.
An energy level diagram helps show this: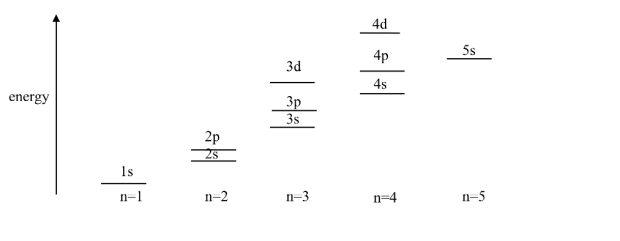 This means the order of filling goes: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d,…
This means the order of filling goes: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d,…
RULE 2) All the orbitals in a subshell must be occupied by a single electron before any of them can have a second electron.
Writing electron arrangements
Electron arrangement are often written using subshell notation, or using electron-in-box diagrams.
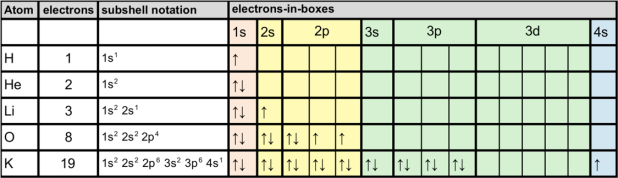
It’s pretty time-consuming filling in the full notation, so sometimes we use the symbol for the Noble Gas at the end of the previous period, to represent the filled shells e.g.
K 1s2 2s2 2p6 3s2 3p6 4s1 is equivalent to [Ar] 4s1
Ca 1s2 2s2 2p6 3s2 3p6 4s2 is equivalent to [Ar] 4s2
Sc 1s2 2s2 2p6 3s2 3p6 3d1 4s2 is equivalent to [Ar] 3d1 4s2 (filling an INNER shell)
N.B. the subshells are normally written in shell order, not in filling order, so 3d is written before 4s, as seen in Sc = 1s2 2s2 2p6 3s2 3p6 3d1 4s2, but the 3d subshell need not be written when it has no electrons in it, so Ca = 1s2 2s2 2p6 3s2 3p6 4s2 rather than 1s2 2s2 2p6 3s2 3p6 3d0 4s2.
Blocks in the periodic table
Elements belong to the s-block, p-block, d-block or f-block corresponding to their highest energy subshell containing electrons:
- Group 1 and Group 2, and Helium are the s-block elements
- In between Group 2 and Group 13 are the d-block elements
- Groups 13-18 (except He) are the p-block elements
- The f-block elements are typically shown at the bottom of the table

Electron arrangements for ions
Starting with the electron arrangement for the corresponding atom, either add electrons (for a negatively charged ion) or remove electrons (for positively charged ions) from the highest energy subshell according to the charge on the ion.
e.g. Ca = 1s2 2s2 2p6 3s2 3p6 4s2 Ca2+ = 1s2 2s2 2p6 3s2 3p6
It is important to appreciate that 4s and 3d are sufficiently close in energy that putting electrons into 3d swaps the relative energies of 3d and 4s, so electrons are removed from 4s before 3d when forming ions of the d-block elements.
e.g. Fe = 1s2 2s2 2p6 3s2 3p6 3d6 4s2 Fe3+ = 1s2 2s2 2p6 3s2 3p6 3d5
Ionisation Energies
Evidence for the arrangement of electrons in shells with different energies comes from experiments in which electrons are progressively removed from atoms. The process of removing an electron is referred to as ionisation, and the energy needed to remove the electron is called the ionisation energy. An atom has as many ionisation energies as it has electrons.
Definition: The first ionisation energy of an element is the energy required to remove one electron from each atom in a mole of atoms of an element in the gaseous state, to form one mole of gaseous 1+ ions.
The symbol is ΔHi, and we add a subscript number to show which ionisation energy we are referring to:
ΔHi1 = 1st ionization energy etc.
We use a balanced equation to show precisely what is happening:
Ca(g) → Ca+(g) + e– ΔHi1 = +590 kJ mol-1
Similarly, the second ionization energy of calcium (energy to remove not two electrons, but to remove one electron from a calcium ion that has already had one removed) can be written as:
Ca+(g) → Ca2+(g) + e– ΔHi2 = +1150 kJ mol-1
(Note that these equations ALWAYS only have one electron in them)
Successive Ionisation Energies
We could keep removing electrons until only the nucleus is left – this would give us the successive ionisation energies of the element. We can use these successive ionisation energies to identify what shells an unknown atom has, and how many electrons occupy each shell.
Definition: The successive ionisation energies of an element are the energies required to remove the electrons successively from each atom in a mole of gaseous atoms of an element, to form one mole of increasingly positively charged gaseous ions.
For example, consider the published successive ionisation energies of sodium:
| Ionisation | Electron Arrangement after ionisation | Electron removed | Ionisation Energy (kJmol-1) |
| 1st | 1s2 2s2 2p6 | 3s1 | 510 |
| 2nd | 1s2 2s2 2p5 | 2p6 | 4,560 |
| 3rd | 1s2 2s2 2p4 | 2p5 | 6,940 |
| 4th | 1s2 2s2 2p3 | 2p4 | 9,540 |
| 5th | 1s2 2s2 2p2 | 2p3 | 13,400 |
| 6th | 1s2 2s2 2p1 | 2p2 | 16,600 |
| 7th | 1s2 2s2 | 2p1 | 20,100 |
| 8th | 1s2 2s1 | 2s2 | 25,500 |
| 9th | 1s2 | 2s1 | 28,900 |
| 10th | 1s1 | 1s2 | 141,000 |
| 11th | – | 1s1 | 158,700 |
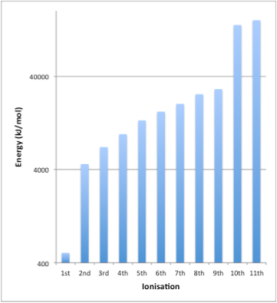 Examining this data we can see
Examining this data we can see
- The energy required to remove electrons gets progressively greater
- There are two ‘steps’ in energy, after the 1st and 9th ionisation
The successive ionization energies reflect the arrangement of electrons in shells, supporting the Bohr model for sodium. The large steps in ionization energy correspond to starting to remove electrons from a principle quantum shell closer to the nucleus.
Using successive ionisation data, we can therefore determine which Group an element belongs to by noting the number of electrons that can be removed before the first large step in ionisation energy. If all the successive ionisations are given, we can also determine which period an element is in, by noting the number of steps in ionisation energy, each corresponding to starting to remove electrons from a new shell closer to the nucleus.
Note: you may be presented with the first ‘n’ successive ionisation energies rather than the full set. Don’t assume the last ionisation energy you are given is for the 1s1 electron!
Factors that affect ionisation energies
- The nuclear charge – the more protons in the nucleus, the more strongly they will attract the electron being being removed.
- Distance from nucleus – the further away the electron to be removed is from nucleus, the weaker the attraction of the nucleus will be.
- Shielding –filled inner shells of electrons shield the electron to be removed, reducing the attraction of between it and the nucleus.
We need to consider all three of these factors when we explain how the attraction between electrons and the nucleus varies, and hence and why ionisation energies vary:
Questions and model answers
i) Explain why there is a large difference between the first and second ionisation energies of sodium.
Sodium has electron arrangement 1s2 2s2 2p6 3s1.
The nuclear charge remains constant as the atom is ionised, but the first electron removed comes from the n=3 shell and is therefore significantly further from the nucleus than the second electron removed. There are also two filled shells (n=1 and n=2) shielding it from the nuclear charge, while the second electron to be removed experiences shielding only from the n=1 shell.
As a result the first electron to be removed experiences much weaker attraction from the nucleus, and therefore takes much less energy to remove.
ii) Explain why there is an increasing trend in the 2rd to 9th ionisation energies of sodium.
Sodium has electron arrangement 1s2 2s2 2p6 3s1.
During the 2rd to 9th ionisations, electrons are being removed from the n=2 principle quantum shell and therefore experience the same shielding from the filled n=1 shell. They also experience the same nuclear charge. However, as electrons are removed from the n=2 shell the repulsion between the remaining electrons in the shell decreases, and the shell contracts, becoming closer to the nucleus.
This means that the attraction between the electron being removed and the nucleus increases, and therefore the energy required to remove the electron increases.